 Latest Trends in Propagation of Aquarium Plants
Latest Trends in Propagation of Aquarium Plants
As the popularity and importance of aquatic plants in the creation of nature aquariums, manicured aquariums, biotopes, etc, are gaining momentum, conventional methods of propagation of these plants are not able to provide the premium quality plants as per the demand. Moreover, the normal propagation methods in open areas always encounter not only the threats from serious pests and diseases but the infestation of snails and algae. Another fact is that such open cultivation systems make it difficult for the farmers to meet the balanced fertilizer program; hence, shelf life is a major issue in the aquarium plant trade.
Tissue culture propagation of these plants is gaining acceptance among serious hobbyists and traders as these plants are available to the end user in plastic containers with its roots well anchored in a balanced nutrient medium. This technique not only enhances the shelf life of the plants (a boon to the sellers) but also can provide the plants in its prime health to end users. Since they are produced in aseptic conditions in laboratories, they are free of algae, snails, and disease.
Moreover, as these plants are grown in a nutrient medium in plastic containers, they are in its robust condition. The initial hiccups of a newly set up tank (not fully cycled) can be withstood by tissue cultured plants, as more reserved food in the plants supports to overcome such non-conducive environment.
Other than employing tissue culture techniques, production is also possible in climatically controlled greenhouses in hydroponic benches. Hydroponic production maintained in such an enclosed area by following balanced fertilizer regime and optimal physical parameters such as humidity and temperature in emergent (above water) conditions can also meet the quality much better compared to open production systems.
Aquatic Plants in Nature/Habitat
The general understanding is that aquatic plants always require water and can grow only in a submerged (underwater) condition which is not always true, as a majority of the plants can adapt to terrestrial and submerged growth as well. This ability is acquired through evolution spanned over millions of years.
 Their habitat is not always inundated; only in the wet season the habitat remains flooded, but during the dry season the water will recede and the habitat will be exposed to dry conditions. As they do not have the ability to move from dry areas to watery areas, they have attained the ability to survive in submerged, semi-submerged or totally terrestrial conditions.
Their habitat is not always inundated; only in the wet season the habitat remains flooded, but during the dry season the water will recede and the habitat will be exposed to dry conditions. As they do not have the ability to move from dry areas to watery areas, they have attained the ability to survive in submerged, semi-submerged or totally terrestrial conditions.
Such aquatic plants which can survive in both conditions are generally known as amphibious plants while those only in aquatic conditions are called true aquatic plants. A majority of the plants in the aquarium trade are amphibious in nature, e.g., stem plants like Rotala, Limnophila, Hygrophila etc, Rosette plants like Crypts, Echinodorus, Anubias,etc and ‘carpet forming’ plants like Hemianthes, Elaeocharis, Glossostigma, etc. are also amphibious plants. True aquatic plants like Aponogetons, Nymphaea, Vallisneria, etc always require water to grow.
Changing Leaf Morphology of Amphibious Plants Depending on Dry and Wet Conditions
Heterophylly, production of different types of leaves of the same plant in underwater conditions and above water conditions always mislead the novices and traders by thinking that they are indeed different plants by misjudging leaf characteristics of the same plant under different growth conditions.
 Underwater growth can result in strikingly red coloration in many plants. The Rotala rotundifolia ‘HRA’, when grown in emergent conditions always produces green to brownish green round shaped leaves with pink stems but in submerged conditions, the same plant produces strikingly red colored, narrow lanceolate (spear-shaped) leaves.
Underwater growth can result in strikingly red coloration in many plants. The Rotala rotundifolia ‘HRA’, when grown in emergent conditions always produces green to brownish green round shaped leaves with pink stems but in submerged conditions, the same plant produces strikingly red colored, narrow lanceolate (spear-shaped) leaves.
Another example of a display of heterophylly is the production of highly dissected leaves in submerged conditions but pointed longer leaves with toothed margins in emergent conditions by Limnophila aquatica. All amphibious plants exhibit different leaf morphology in aquatic and non-aquatic conditions as a means to survive.
Underwater, round and comparatively thicker emergent leaves will get damaged due to strong water current, so streamlining in the form of pointed elongated thinner leaves or highly dissected leaves is mandatory for its survival. The underwater leaves are very tender and lack a wax coat (cuticle) on its surface, so strong solar radiation doesn’t impede the photosynthetic machinery. The presence of more color pigments like anthocyanins and carotenoids can protect the photosynthetic machinery of leaves from being damaged due to high light intensity in open water bodies. As this ability is acquired during the course of evolution, depending on the intensity of light; the color will be brighter and brighter.
Propagation Systems
As I mentioned above, progressive farmers exploit sophisticated farming techniques such as Tissue Culture, Hydroponics, or even Aeroponics for commercial-scale propagation of aquatic plants including liverworts (Riccia, Riccardia, etc) and mosses.
 The advantages are:
The advantages are:
- Strictly following a fertilizer regime.
- Strictly monitoring the light, temperature, and relative humidity.
Tissue Culture or Micropropagation or In vitro Propagation
Tropica Aquarium Plant Pte. Ltd, Denmark is a pioneer in employing this technique to support greenhouse production of aquarium plants in the late 1980s. Later, Oriental Aquarium Pte Ltd. Singapore adopted this technique to support the hydroponic cultivation of aquarium plants.
I was fortunate enough to associate with Oriental Singapore, developing viable protocols for the production of aquatic plants by tissue culture. During my association with Singapore firm for more than a decade, I was able to sharpen my skills in the area of modern agricultural practices.
Later the attempt of introducing tissue cultured or in vitro plants directly into the aquarium by eliminating the process of hardening in the greenhouse became very successful. Now world leading aquarium plant producers are successfully employing this technique to supply the plants in vitro conditions to customers.
Stages of Micropropagation
Selection and Disinfection of Explant
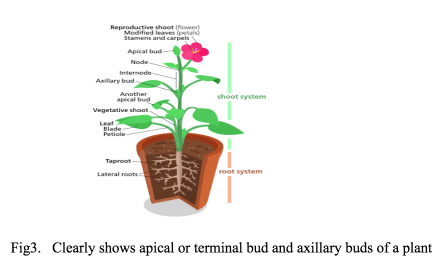
An explant in tissue culture procedure can be actively growing or dormant buds such as apical buds and axillary buds or actively dividing cells within the buds (meristem) or even viable young cells in any part of the plant. Totipotency, the ability of every cell in the living organisms capable of developing in to complete organisms is largely exploited in plant tissue culture.
Selection of explants is always very important for the production of healthy and robust plants.
- Generally, shoot tip enclosing the apical bud or dormant buds in the axes of the leaves are used as explants for the propagation of aquatic plant by tissue culture
- The explants should be selected from a healthy and vigorously growing plant in emergent conditions, free of diseases and pest infestation, as this selection process will help the production and maintenance of healthy mother cultures for commercial production.
Disinfection of Explants
As we select the explants from plants growing in greenhouse conditions, it can harbor thousands of bacterial and fungal organisms including its spores. Even though they are non-pathogenic in nature, it can proliferate vigorously on the nutrient-rich tissue culture medium and interferes the growth of less established explants on the medium. Disinfection is mandatory for the successful establishment of explants on sucrose enriched nutrient medium.

- Under non aseptic conditions (normal conditions,) the selected explants (shoot tip and axillary buds) should be thoroughly washed with detergent solutions several times after removing unwanted plants parts such as fully opened and partially opened leaves.
- After washing with soap solution, a dip in 70% ethyl alcohol for 1 minute followed by another wash with distilled water or R O water is really helpful to bring down the microbial population in the explants.
- Aseptic conditions can be achieved in a sterile cabinet generally known as Laminar Flow Hood (LF). Such a cabinet can provide a sterile environment by pumping sterile air through HEPA filter (very efficient in removing microbe in the air).
- All materials meant for obtaining clean explants (initiation process) such as tools (forceps, scissors, scalpel, etc) water, conical flasks and the plates should be sterilized by steam sterilization in an autoclave at 15psi with a temperature of 120C for minimum 20 minutes before being taken to sterile cabinet or laminar flow hood.
- A strong but safe disinfectant to be used to disinfect explants on the sterile hood. Generally, commercial bleach under the trade name CLOROX is very effective in stage wise disinfection procedure
- Transfer the explants to a conical flask with 15% Clorox solution (diluted with sterile water) and add a drop of surfactant Tween 20 in order to effect disinfection with a great rate of success by eliminating the surface tension of water. Duration of first stage sterilization should be standardized through trial and error methods. 10-15 minutes incubation is generally recommended.
- Transfer the explants to a sterile plate after rinsing with sterile water a minimum of 3 times and remove the outer whorls of dead tissues or leaf primordia (very young leaves closely adhering to meristem) by placing them on a sterilized plate or Petri dishes.
- Continue the same procedure by decreasing the strength of the disinfectant solutions to 10% and then to 5% for an incubation period of about 5-10 minutes.
After the 3 stages of disinfection, the final size of the explants is less than 0.2 cm. Definitely, some skill is called for to obtain such small size explants without killing the meristem. As aquatic plants are notorious for internal contaminants or endophytic microbes, the size of the explants is very important to obtain clean cultures.
 The explants after disinfection are incubated in sterilized tissue culture medium dispensed in test tubes for 2 weeks. The success rate of obtaining clean cultures for a skilled technician is about 40-60%. After 2 weeks of incubation in test tubes, the clean explants, or initiates, are transferred to bottles containing sterilized medium favoring shoot multiplication.
The explants after disinfection are incubated in sterilized tissue culture medium dispensed in test tubes for 2 weeks. The success rate of obtaining clean cultures for a skilled technician is about 40-60%. After 2 weeks of incubation in test tubes, the clean explants, or initiates, are transferred to bottles containing sterilized medium favoring shoot multiplication.
Tissue Culture Medium, Preparation, and Sterilisation
Tissue Culture Medium: Murashige and Skoog medium popularly known as MS medium is used as basal medium (all other constituents present except hormones) for tissue culture propagation of aquarium plants. The composition of this medium is freely available on the internet. This medium enriched with 3% sucrose which acts as the source of energy for the growing explants. Even though this well-known nutrient medium can be used for the majority of aquarium plants by adjusting its strength as full strength MS medium (original concentration),1/2 strength MS medium (half the original concentration) or 1/3 strength MS medium, it cannot be regarded as a universal medium for aquarium plants. Plants like liverworts and mosses require diluted tissue culture formulations like Knops or White’s medium for its successful production. Standardization of nutrient medium can be achieved by trying different strength of M S medium and other formulations as mentioned above.
 Growth Regulators / Plant Hormones: Auxins and Cytokinins are 2 major groups of hormones and its relative concentration in nutrient medium determines the fate of cultures. Higher concentration of Cytokinin favors shoot multiplication, but root growth is determined by higher levels of Auxin. So, cytokinin is generally known as shoot promoting hormone while Auxin as root promoting hormone.
Growth Regulators / Plant Hormones: Auxins and Cytokinins are 2 major groups of hormones and its relative concentration in nutrient medium determines the fate of cultures. Higher concentration of Cytokinin favors shoot multiplication, but root growth is determined by higher levels of Auxin. So, cytokinin is generally known as shoot promoting hormone while Auxin as root promoting hormone.
BAP (6 Benzylaminopurine or Benzyladenine) is the major cytokinin used in the aquarium plant tissue culture to promote shoot multiplication. As it is a stronger hormone, less strong cytokinin like Kinetin is provided to sensitive plants in order to avoid abnormal multiplication. Usual concentrations vary from 0.1 mg/l to 2mg/l
Alpha NAA (Naphthalene acetic acid) or IBA ( Indole butyric acid) is generally used for promoting root growth. In a majority of the cases shoot cultures of aquarium plants develop root when they are transferred to a nutrient medium devoid of hormones as endogenous auxin (auxin present in the plant) is sufficient enough to trigger root growth. Only in cases no root growth is observed root-promoting hormones to be added at the concentrations of 0.1mg/l to 0.5 mg/l. IAA (Indole Acetic Acid) a natural auxin can be used for root development. However, this auxin is photolabile (degraded under light), so its stock solution is to be kept in amber colored bottles.
 Media Preparation: As the plant tissue culture medium contains many chemicals and is difficult to weigh the individual constituents every time, the corresponding stock solutions are prepared. The concentration of stock solutions may be 50x (50 times higher the original concentration), 100x (100 times higher the original concentration) or even 1000x. Information regarding stock preparation is available on the web.
Media Preparation: As the plant tissue culture medium contains many chemicals and is difficult to weigh the individual constituents every time, the corresponding stock solutions are prepared. The concentration of stock solutions may be 50x (50 times higher the original concentration), 100x (100 times higher the original concentration) or even 1000x. Information regarding stock preparation is available on the web.
Hormone stock is prepared as mg/l stock or ppm (parts per million) stock or in milligram molecular weight (mM stock). The information can be found elsewhere.
Optimizing Nutrient Media: Media formulations may vary from plant to plant. Standardization of medium for optimal growth is a major challenge for the production of plants by tissue culture. A novice can begin with MS medium at different strength by adding cytokinin such as BAP of about 0.1 to 0.5 mg/l BAP. In a majority of the cases, shoot multiplication can be achieved. If not, you can try higher concentrations of BAP. Once the normal multiplication rate is achieved (a clump of 4-6 plants in the case of rosette and stem plants and uniform coverage of healthy runners for ‘carpet forming’ plants), the shoots are transferred into another medium containing only auxin such as NAA or IBA of about 0.25-0.5 mg/l to initiate healthy root development. Generally, this combination with root-promoting hormone is done in sterilized plastic containers.
 After an incubation period of about 3-4 weeks, these containers will be ready for sale. Generally ‘Carpet forming’ plants require one media combination. The same combination for ‘carpet forming’ plants can be used for stocking mother cultures in bottles and also the production of plants in plastic containers for sale. In certain cases activated charcoal powder is incorporated into a medium at the rooting stage for the healthy production of plants. Charcoal helps in regulating the supply of nutrients to the plants over a longer period.
After an incubation period of about 3-4 weeks, these containers will be ready for sale. Generally ‘Carpet forming’ plants require one media combination. The same combination for ‘carpet forming’ plants can be used for stocking mother cultures in bottles and also the production of plants in plastic containers for sale. In certain cases activated charcoal powder is incorporated into a medium at the rooting stage for the healthy production of plants. Charcoal helps in regulating the supply of nutrients to the plants over a longer period.
The liquid medium prepared by using various stock solutions should be made up to the final volume with distilled water or ultrapure water and pH of the medium is adjusted to 5.8. In order to obtain a semisolid medium, agar is to be added at about 6g/l.
Sterilization of Medium: The nutrient medium after the addition of agar should be dispensed into glass bottles or plastic containers while being stirred continuously to maintain uniform dispersion of agar. A magnetic stirrer can be used for the continuous stirring. After dispensing into bottles or containers, they should be sterilized by employing steam sterilization technique. To achieve steam sterilization, an autoclave is used. The sterilization can be achieved at 15psi (115C) for about 15 minutes. If containers are already sterilized by Gamma radiation or other means, the media dispensing should be done under sterile conditions. The use of media sterilizers for the sterilization of tissue culture medium in bulk quantity coupled with media dispensing device makes it possible for dispensing sterilized medium into pre-sterilised empty plastic containers under sterile/ aseptic conditions in laminar flow cabinets.
Maintenance of Cultures: Plant cultures are regularly transferred to fresh nutrient medium under aseptic/sterile conditions; a process known as subculturing. This way, we can increase the quantity of mother stocks. The multiplication ratio varies from plants to plants. For example, carpet forming plants can yield 4 bottles if one bottle of culture is subcultured. In general 3-5 bottles of cultures can be produced from one bottle. At the time of subculture, trim the roots and leaves of plant clumps and split it into 3 -4 pieces in the case of stem and rosette plants and for ‘carpet forming plants’ the runners should be cut into 1 cm long pieces after trimming the leaves and roots. The yield is very high for ‘carpet forming plants’.
The interval between two subculture is known as subculture period and it varies from 4weeks (fast growing plants) to 6 weeks (medium growing plants) Regular subculture is essential for marinating vigor of the plants and multiplication rate. If we use the plants from bottles without regular culture (delayed subculture) for plastic container production, quality will be affected and you can expect lots of complaints from the end user.
The cultures are generally maintained in climatically controlled growth rooms by providing optimal light (3000-5000 lux) and temperature at about 23-26C. The photoperiod (lighting period) can be adjusted to 12-14 hours with the help of a timer for a day.
Hydroponic Cultivation
Majority of the aquarium plants can be propagated by hydroponics in modern greenhouses with an automated climatic control system. Here we exploit the ability of aquatic plants to grow in above water (emergent) conditions. Growth medium can be soilless such as Rockwool (horticultural grade), Cocopeat or Polyurethane Form. The plants are placed in an artificial medium and put in netted pots. The planted pots in hydroponic trays are transferred to hydroponic troughs where nutrient solution is circulated in a semi-continuous manner with the help of a timer.
Nutrient solution should be well balanced by incorporating all required elements for optimal plant growth. As groundwater has high EC (electrical conductivity) and an alkaline pH (>7) due to the presence of high Calcium, Carbonate, and bi Carbonate the water should be purified by using appropriate purification system. In general Reverse Osmosis process is utilized as it can remove more than 95% of the total salts present in the water. The water treated with R O system help maintain the correct EC (~1200 us) with pH between 6 and 7 after the addition of nutrients
Now biological fungicides and biological pesticides are available in the market. Application of these environment-friendly organisms together with maintaining standard hygienic conditions can eliminate the use of toxic chemicals to eliminate pest and diseases problems in greenhouse production
A modified version of Aeroponics is used to cultivate mosses, ferns, and Anubias on driftwood and rocks. The nutrient solution having low EC (< 200us ) is sprayed in the form of fine fog at high pressure (>80psi) at small intervals. As the fog is composed of particles less than 10micron size, it will remain suspended in the air for a longer period and gradually moves down. This results in a high humidity environment and also providing enough nutrients for plants growing in this system.
Article by: Dr. S K Unnikrishnan
Photos: Dr. S K Unnikrishnan
About the Author, Dr. S K Unnikrishnan, Aquatic Plant Specialist, well accomplished aquatic plant grower with an experience of 30 years. Associated with Oriental Aquarium Pte Ltd, Singapore as Research Scientist for more than a decade and later joined as Project Head, SWA-SBL joint Venture, Bangalore, India which produces tissue cultured aquarium plants under the brand ADA Japan. Currently working as a consultant. E-mail unni_sk@hotmail.com
 Biotope One A Study of Flora and Fauna
Biotope One A Study of Flora and Fauna 

